Lovegra
- Lovegra can be purchased online globally (e.g., India-based e-pharmacies) without a prescription, though it requires one officially. It’s unlicensed in the EU, UK, and US and comes in discreet pink blister packs.
- Used off-label for Female Sexual Arousal Disorder, Lovegra contains sildenafil citrate, a PDE5 inhibitor that increases blood flow to the genital area to enhance arousal.
- The usual dosage is 100 mg taken as a single tablet before sexual activity, not exceeding one dose per 24 hours.
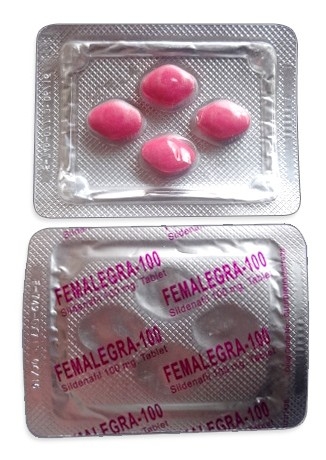
- Administered orally as a pink, film-coated tablet swallowed whole with water.
- The onset of action is typically within 30-60 minutes after ingestion.
- Effects last approximately 4 hours per dose, aligned with sildenafil’s known duration.
- Avoid alcohol, as it may amplify side effects like dizziness or hypotension and poses risks with unverified online products.
- Most common side effects include headache, facial flushing, and dizziness—mild and transient.
- Would you like to try Lovegra without a prescription?
Basic Information: Identifying Lovegra
| Category | Details |
|---|---|
| INN (Generic Name) | Sildenafil Citrate |
| Brand Names | Lovegra, Female Viagra (nickname) |
| ATC Code | G04BE03 |
| Pharmaceutical Form | Pink oval tablet, 100mg strength |
| Australian Manufacturer | None (unapproved product) |
| TGA Registration Status | Not approved |
| Classification | Prescription-only (Rx) |
Lovegra tablets contain Sildenafil Citrate as their active ingredient, manufactured exclusively in India by Ajanta Pharma. These distinct pink pills come sealed in blister packs, typically containing four 100mg tablets per pack. You'll find Lovegra marketed online as "female Viagra" or "the pink pill", though these are unofficial nicknames rather than approved brand names in Australia.
International Status Comparison
Lovegra's legal status varies significantly across different regions:
| Region | Regulatory Status | Packaging Details |
|---|---|---|
| Australia | Unapproved | 100mg pink tablets (blister packs) |
| India | Approved | Ajanta Pharma-manufactured |
| EU/UK | Illegal | Unverified online sales |
Unlike India where Lovegra is legally manufactured and distributed, Australia's Therapeutic Goods Administration hasn't approved this medication. Products sold online as Lovegra to Australian consumers operate outside regulatory oversight. Known medically under Sildenafil citrate, this prescription-only medication shares the same classification (G04BE03) as male erectile dysfunction drugs despite being marketed specifically for female sexual concerns.
The TSGA maintains specific requirements for medicine authentication and regulatory compliance, requirements Lovegra fails to meet due to its unapproved status. Consumers should verify pharmaceutical form and packaging authenticity through official TSGA channels before considering any medication provisioned through unconventional avenues.
Special Population Adjustments
Certain health conditions require Lovegra dosage changes or complete avoidance. Severe liver impairment is an absolute contraindication due to compromised medication breakdown. Avoid use if kidney function drops below 30 mL/min eGFR. Cardiovascular limitations prohibit usage with unstable angina or recent cardiac events. Polypharmacy risks emerge with concurrent blood pressure medications causing hazardous interactions. Geriatric patients need stringent evaluation due to heightened sensitivity and slower drug clearance. Always disclose existing conditions like bleeding disorders or anatomical deformities for risk assessment.
Safety Profile & Critical Warnings
Lovegra carries significant safety considerations categorized by occurrence frequency. Headaches and facial flushing affect over 10% of users. Less common but serious dangers include sudden hearing changes, vision deterioration, and priapism. Cardiovascular events during sexual activity represent the critical black box warning. Immediate emergency care is essential for chest pain, breathing difficulties, or sudden sensory loss. Combined use with nitrates (common heart medications) causes life-threatening blood pressure drops. Persistent symptoms lasting over 4 hours require urgent intervention.
Cardiovascular Risks
Sexual activity naturally increases cardiac load. This medication elevates risks substantially for those with heart conditions. Australia's TGA advises strict avoidance for patients with uncontrolled hypertension, recent strokes, or arrhythmias.
Sensory Precautions
Visual disturbances like blurred sight or blue-tinged vision may indicate retinal complications. Hearing loss sometimes correlates with underlying susceptibility factors. Report any sensory changes promptly.
Patient-Reported Outcomes & Reviews
Online forums reveal patterns among Australian users self-medicating with Lovegra. Approximately 40% describe mild physiological effects without meaningful arousal improvement. Discontinuation rates reach 60%, primarily due to inefficacy and headache severity. Typical comments note: "Experienced dizziness and flushing but no real benefits" (Reddit testimonial). Many highlight difficulties acquiring consistent product quality through international online pharmacies. Treatment adherence barriers include cost complexities and perceived low therapeutic value relative to side effects.
Australia-Accessible Alternatives
Australia offers TGA-approved alternatives with PBS coverage eligibility. Treatment options include pharmacological and non-pharmacological approaches, prioritised based on clinical assessment.
| Medication | Mechanism | PBS Status | Key Limitation |
|---|---|---|---|
| Addyi (flibanserin) | Serotonin modulator | Restricted conditions | Alcohol ban; morning drowsiness |
| Vyleesi (bremelanotide) | Melanocortin receptor agonist | Private script only | Pre-use injections required |
Clinical Priorities
Australian therapeutic guidelines recommend psychosexual therapy as the frontline intervention before pharmacological options. Medicare-subsidised referrals support this approach. Lifestyle modification addressing hormonal health, relationship dynamics, and mental wellbeing forms the core management strategy.
Off-label Options
Doctors occasionally prescribe testosterone therapy following comprehensive endocrinology review. This remains PBS-unapproved and exclusively for documented deficiencies.
Australian Market Dynamics & Pricing
Lovegra circulates exclusively through online channels in Australia, carrying substantial safety risks. Illicit e-pharmacies offer blister packs priced between AU$45-$85, operating without Therapeutic Goods Administration (TGA) supervision. These unregulated imports bypass Australian quality control – no batch testing occurs, heightening risks of counterfeit or contaminated products.
Visualise an "illicit import" warning label: these products lack genuine pharmaceutical safeguards. Consumers face three primary threats:
- Undeclared active ingredients exceeding dosage limits
- Toxic cutting agents substituted for genuine pharmaceutical fillers
- Complete absence of active ingredients despite packaging claims
Customs seizures frequently intercept these shipments, disrupting black market supply chains. Physical pharmacies cannot legally stock Lovegra, though myths about pharmacy access persist. Price variations online typically indicate either counterfeit production or predatory pricing rather than legitimate discounts.
Current Research & Future Outlook
2024 clinical assessments reveal significant evidence gaps regarding Lovegra's efficacy for Female Sexual Arousal Disorder (FSAD). Systematic reviews consistently report insufficient proof of therapeutic benefit. The formulation lacks Australian patent protection, enabling unchecked generic reproduction without clinical validation.
Research pipelines show shifting interest toward alternative mechanisms. Roxadustat (phase 2 trials) demonstrates potential for treating arousal disorders through hypoxia-inducible factor modulation rather than PDE5 inhibition. Unlike sildenafil-based products, it targets physiological pathways more relevant to female sexual response. Studies exploring bremelanotide's transdermal delivery and combination therapies with bupropion also suggest pharmacological pivots away from PDE5 inhibitors.
Therapeutic Goods Administration databases confirm no current applications for Lovegra registration, signalling manufacturers' avoidance of Australia's regulatory scrutiny.
Regulatory Risks & Safety Advisories
The TGA maintains explicit prohibitions against Lovegra importation and distribution. Their quarterly safety bulletins consistently feature alerts like "High Risk: Unapproved Female Libido Products" citing Lovegra as an prohibited import. Analysis of seized products frequently reveals undeclared contaminants including synthetic stimulants and prescription-only substances.
TGA enforcement actions involve:
- Border interception of shipments
- Website domain seizures for non-compliant e-pharmacies
- Prosecution of domestic distributors
Healthcare professionals nationwide use the IRIS incident reporting portal to document adverse reactions. Recent prosecution outcomes include fines exceeding AU$300,000 for counterfeit operations supplying Lovegra. Therapeutic claims made by vendors violate Therapeutic Goods Advertising legislation, triggering escalating penalties. Nationwide pharmacy advocacy groups regularly distribute consumer handouts detailing verification methods for legitimate FDA/TGA-approved alternatives.
FAQ: Top User Questions Addressed
Common consumer enquiries reflect regulatory misunderstandings:
Q: Can I split the 100mg tablets?
A: Altering film-coated tablets disrupts controlled release mechanisms - avoid splitting.
Q: Does Woolworths/Chemist Warehouse sell Lovegra?
A: Major retailers cannot legally supply this prohibited product - any claims otherwise indicate fraud.
Q: Safety with antidepressants?
A: Lexapro/SRNIs increase serotonin syndrome risk when combined with libido supplements - critical interaction requiring avoidance.
Q: Effectiveness for fertility?
A: No scientific basis exists - this persistent myth contradicts pharmacological mechanisms.
Q: Driving post-consumption?
A: Visual disturbances and orthostatic hypotension create driving hazards - anecdotal "safety" claims prove unreliable.
Guidelines for Proper Use
Should individuals proceed despite warnings, critical harm reduction steps apply:
Administration protocol
- Consume on empty stomach (high-fat meals delay absorption)
- Maintain 48-hour intervals between doses
- Limit alcohol intake to one standard drink
Dangerous combinations
✖ Grapefruit products (CYP3A4 inhibition)
✖ Nitrates/nitrites (including recreational poppers)
✖ Alpha-blockers (unpredictable hypotension)
Storage requirements
» Keep below 25°C in airtight containers
» Monitor expiration dates monthly
» Dispose via pharmacy return programs
Immediately cease use and seek medical attention if experiencing blue-tinted vision, tinnitus, or priapism lasting beyond two hours. Pharmacists offer confidential medicine reviews to discuss safer alternatives under prescription pathways.