Proair Inhaler
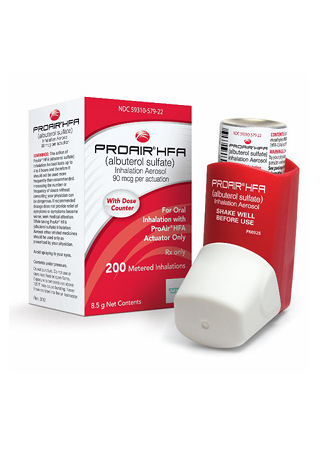
Proair Inhaler
- Proair Inhaler requires a prescription, but our pharmacy provides it without one — fast shipping to Australia in discreet packaging.
- Proair Inhaler (albuterol/salbutamol) treats asthma and COPD by relaxing airway muscles through selective beta₂-adrenoreceptor activation for bronchodilation.
- Usual dose: 2 inhalations (100 mcg each) every 4–6 hours as needed, or before exercise.
- Administered via metered-dose inhaler aerosol.
- Starts working rapidly within 5–15 minutes.
- Effects last 4–6 hours.
- Avoid alcohol as it may worsen breathing symptoms.
- Common side effects: Tremor, headache, tachycardia, nervousness, dizziness, muscle cramps, or sore throat.
- Ready to experience relief with Proair Inhaler — bypass the prescription hassle and order yours now?
Basic Proair Inhaler Information
| Information Type | Details |
|---|---|
| Active Ingredient | Salbutamol (International Nonproprietary Name) |
| Brand Names | ProAir® HFA, Ventolin®, Respirol®, generic salbutamol |
| ATC Code | R03AC02 (Selective β2-adrenergic agonists) |
| Formats Available | Metered-dose inhaler (100-108 mcg per actuation) |
| Manufacturers | Teva Pharmaceuticals, GlaxoSmithKline (Ventolin®), various generics |
| Australian Registration | Therapeutic Goods Administration (TGA)-approved |
| Classification | Prescription-only (Rx) medicine |
The Proair inhaler contains salbutamol, known internationally as the active bronchodilator ingredient in rescue inhalers. Identified medically by its ATC code R03AC02, this prescription medication falls into the category of fast-acting beta-agonists. Traditional packaging includes aluminium canisters providing 200 metered doses, featuring integrated dose counters to help patients track remaining medication. ProAir HFA has been discontinued by Teva Pharmaceuticals in Australia, though identical generic equivalents remain available nationwide. As a Schedule 4 poison, obtaining it requires a valid prescription under Australian pharmaceutical regulations.
Understanding Proair's Mechanism and Interactions
Proair works through targeted activation of beta2-adrenergic receptors in the lungs' bronchial muscles. When inhaled, salbutamol triggers relaxation of these muscles within 3-5 minutes, rapidly opening airways during asthma attacks. Therapeutic effects typically last 4-6 hours, suitable for acute symptom management. Metabolism occurs primarily in the liver through CYP450 enzymes.
The majority (76%) of unchanged salbutamol exits the body through renal excretion. Patients should exercise caution with combined use of certain antidepressants (MAOIs, TCAs) and diuretics, which may enhance cardiovascular risks or cause hypokalemia. While alcohol isn't strictly contraindicated, it might intensify side effects like tremors. Anyone experiencing irregular heartbeat should seek medical evaluation.
Medical Uses Across Patient Groups
Proair holds TGA approval for two primary respiratory indications:
- Acute asthma symptom relief: Rapid intervention during asthma attacks
- Exercise-induced bronchospasm prevention: Pre-treatment before physical activity
Special Populations Considerations
Off-label COPD usage occurs under specialist supervision for symptom management. Children aged ≥4 years follow identical dosing protocols to adults. During pregnancy, TGA classifies Proair as Category B1, indicating safety demonstrated in limited human studies. Usage requires careful benefit-risk assessment until further data confirms absolute safety. Geriatric patients require cardiac monitoring due to susceptibility to cardiovascular effects, without routine dosage modification.
Australian Availability Considerations
Following ProAir HFA's discontinuation by Teva, Australians access equivalent generic salbutamol inhalers containing identical medication formulations. Pharmacists can advise on comparable devices maintaining the standard 100-108mcg per dose deliverability. Prescriptive authorization remains mandatory nationwide - no emergency non-prescription supply exists legally despite medication urgency scenarios. Travellers must retain prescriptions and maintain devices within 15-25°C storage conditions.
##